CoronaVax safety in the Netherlands: Update 2
Further update of Theo Schetters' survey of public data from the NL
As some readers may recall, Jill and I traveled to Belgium in June of this year to meet with Drs. Mattias Desmet, Theo Schetters and many other key opinion leaders from that region, participated in meetings, and recorded podcasts aimed at a European audience. During that time, I recorded a podcast with Theo and Marlies Dekkers which focused on the mRNA Coronavirus genetic vaccines. To reduce the risk of censorship and deplatforming, that podcast was uploaded to the Substack servers, and together with an accompanying article can be found here.
For those who do not know Dr. Schetters, he is an exceptional, gifted, and highly respected vaccinologist. Dr. Theo Schetters obtained a PhD in Medicine from Nijmegen University in the Netherlands, and received a visiting scientist award from the Royal Society (London) to work on malaria immunology at the National Institute for Medical Research in Mill Hill, London (UK). From 1988 to 2014 he worked at Intervet International (Boxmeer, The Netherlands) where he developed a vaccine against coccidiosis in chickens (Nobilis® Cox ATM) and a vaccine against Babesia infections in dogs (Nobivac® Piro). He is inventor of an improved vaccine formulation against diseases associated with Rhipicephalus ticks. Presently, he is director of ProtActivity, a company that focuses on vaccine development against ticks and tick-borne protozoal infections. In 2004 he received the Medal of Honour of the Faculty of Pharmacy, University of Montpellier 1 in France and was bestowed Professeur Invité as recognition for his contribution to longstanding collaborative research with the Laboratory of Cellular and Molecular Biology of the University (head Prof. Andre Gorenflot). He is an editorial board member of “Veterinary Parasitology, Trials in Vaccinology” (Elseviers Science Publishers) and “Parasitology” (Cambridge University Press).
Prior substack essays covering the Coronavax safety findings of Dr. Schetters can be found at the following locations:
CoronaVax safety in the Netherlands: Dr. Theo Schetters' survey of public data from the NL and Ontario, Canada
Update: CoronaVax safety in the Netherlands: An update of Theo Schetters' survey of public data from the NL
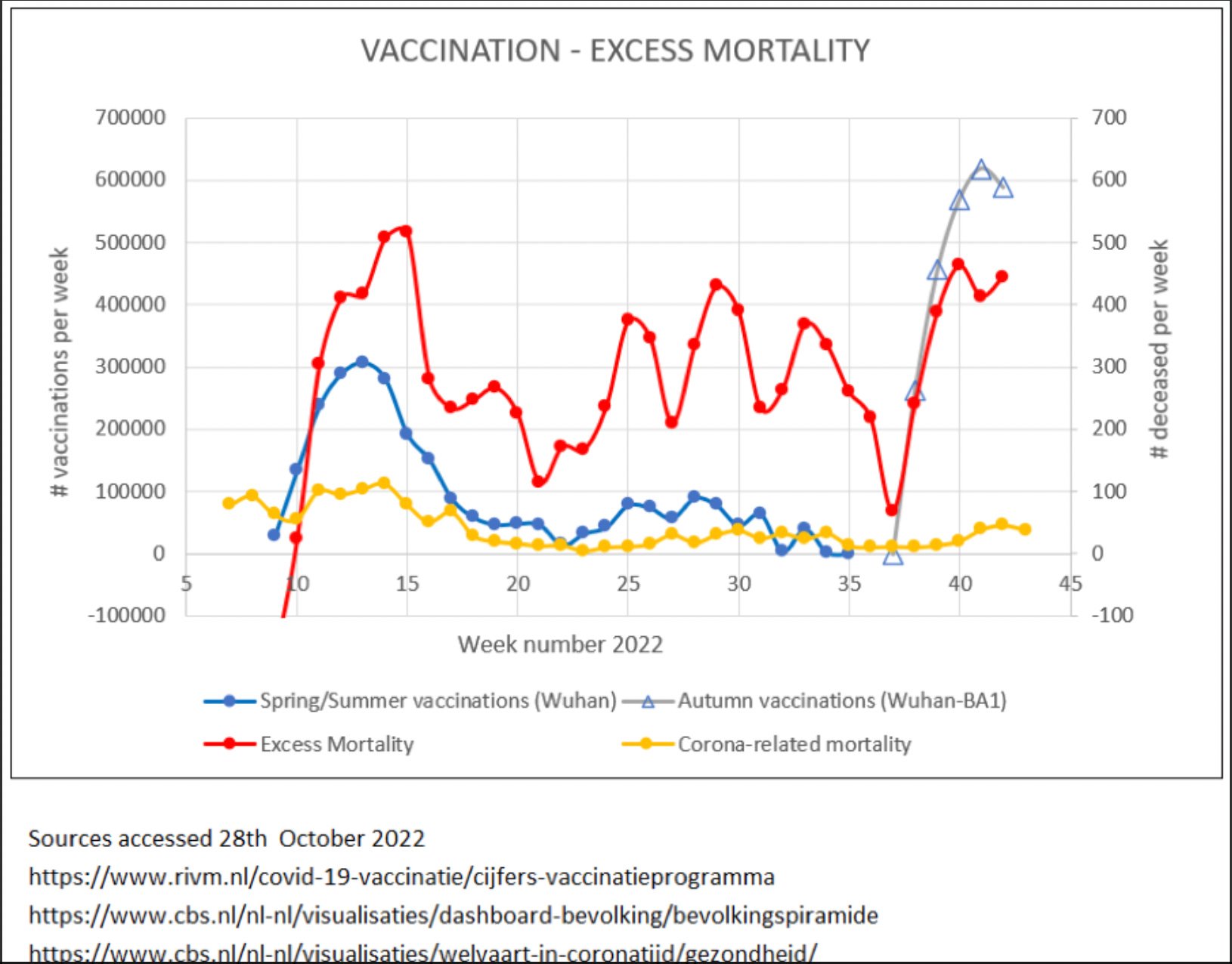
This essay is the fourth in the series detailing Dr. Schetters work analyzing public health data from the NL, with a focus on potential temporal (time) correlations between vaccination and excess all cause mortality in the high risk elderly cohort.
In the Netherlands, the autumn vaccination campaign started in week 37 of 2022 (triangles). As scheduled, the elderly (>80 years old) and subjects that belong to the high-risk population, were the first to receive the vaccinations (mRNA corona vaccines). Immediately after the onset of the campaign, there was a rise in mortality that followed the dynamics of the vaccinations (red line). This temporal relationship between vaccination and increase in mortality appeared similar to that found earlier during the spring vaccination campaign that was started in week 9 earlier this year (blue line). Importantly, the mortality with/from SARS-CoV-2 was low, especially during the autumn vaccination campaign. Although the data lack sufficient detail to firmly establish causality, these results call for immediate suspension of the vaccinations. after this initial analysis, the government was warned that detailed investigation into the cause of increased mortality was of paramount importance.
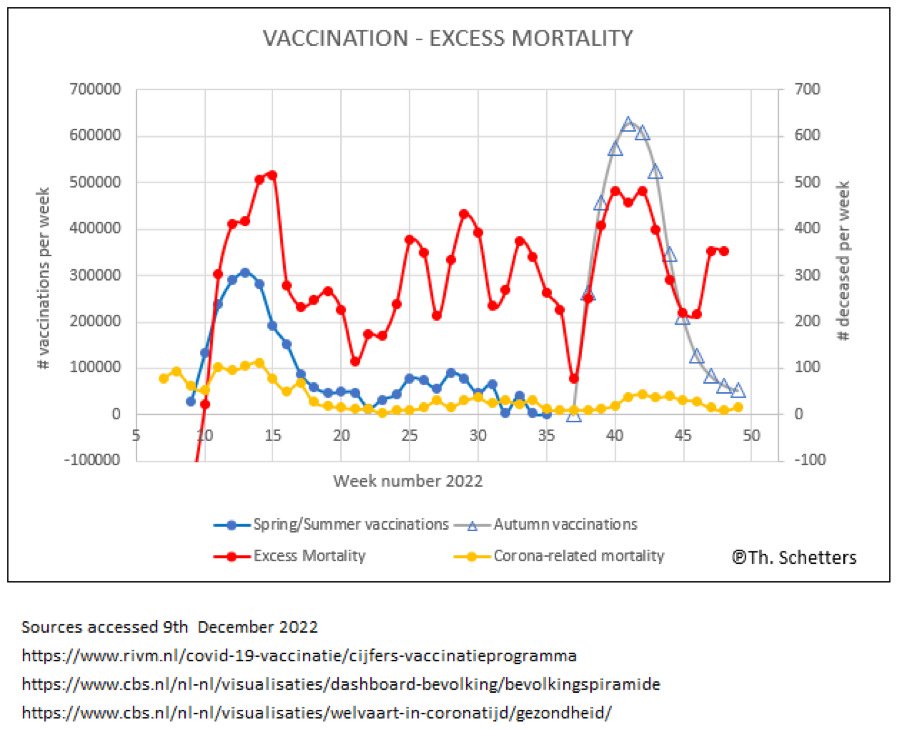
UPDATE CORRELATION VACCINATION AND MORTALITY IN THE NETHERLANDS OCTOBER 30TH 2022
A presentation that explains which data were used, where they can be found and how they were analyzed can be found here.
Unfortunately, neither investigation nor action was undertaken. Now Dr. Schetters has updated this analysis with additional recent data, and as feared the correlation appears even stronger.
Finally, as I have been warning everyone - the FDA has many, many mRNA vaccine clinical trials approved. The mRNA COVID-19 vaccine was just the beginning…
Trial Site News, Dec 13, 2023
“The United States Food and Drug Administration (FDA) just granted Fast Track designation for Pfizer and BioNTech’s messenger ribonucleic acid (mRNA)-based combination vaccine candidate against COVID-19 and influenza. This product, which is currently experimental, is supposed to prevent the two respiratory conditions via a single injection. Fast Track is a process designed to facilitate development and expedite the review of new drugs and vaccines intended to treat or prevent serious conditions and address unmet medical need. Importantly, there are only so many resources in the FDA to go around. With Pfizer and BioNTech securing access to such a program it’s to the detriment of some other biotech or pharma companies which may or may not be warranted based on the underlying details of what might be excluded. The current combined influenzas and SARS-coV-2 experimental vaccine is based on their existing Omicron bivalent vaccine that includes mRNA strands encoding the wild-type spike protein of SARS-CoV-2 and the spike protein of the Omicron sublineages BA.4/BA.5. But those sublineages represent now under 15% of all U.S-based infections! BQ.1 and BQ.1.1 have surged now, representing about 70% of cases. A few studies that TrialSite have tracked thus far suggest subpar performance of the current bivalent booster vaccine against these now predominant subvariants. The underlying strategy seems to benefit Pfizer’s convenience but not the safety of the public. It’s a simple game. Reuse and bundle the same technology—regardless of how good it really is—and make a lot of money.”
Pfizer is playing a game with the government and the public—or maybe the company and the government are playing a game with the public. Leverage the momentum of COVID-19, exploit existing subpar vaccine products, and make even more money on a combined product. Of course, the company doesn’t disclose that the death rates associated with Omicron, primarily an upper respiratory infection, are far lower, and that the current product durability is challenged to say the least.
Today I am in Austria for an event. I have been informed by one of the scientists here that this expedited plan has been approved in Austria and the EU also. Although so far I have not been able to find a link on the Internet confirming this information. If it is true, this is more evidence that the coordinated effort by world governments to have a one world health – a plan that weaponizes public health across the world against its peoples continues.
We live in a world of many different cultures, environmental conditions, genetic diversity, socio-economic status, governmental structures and health care systems. The idea of a one size fits all model for health care delivery, including for infectious disease outbreaks, is naive at best and most likely driven by the profit making machines of big pharma. Regulatory capture of governments and NGOs, such as the World Health Organization continues at a break-neck pace.