Nobel Prize for Physiology or Medicine: Factcheck
Facts are stubborn things, and "official" propaganda is still propaganda
So, it finally happened. And with the announcement came yet another wave of troll and bot attacks on my credibility. Coupled with social media calls for me to comment on the event. Commenting on which I am generally not comfortable with, because I am not prone to pettiness. Jill and I gave ourselves a day to just breathe, discuss the meaning and implications, and process and respond to the many kind, supportive comments and condolences from friends and colleagues from all over the world.
First off, congratulations to Drs. Kariko and Weissman. This award comes on top of having received the Lasker award, and similar awards from the governments of Spain and Israel.
A day in, I think that the proper way to approach commenting on this is to examine the truthfulness of the official statements made by the Nobel Committee concerning their decision.
First off, the core position that has been taken is to not assert that Kariko and Weissman were the originators or inventors of the use of mRNA to elicit an immune response (vaccination). I suppose that there is a small modicum of validation in that. As I have often had to explain in so, so many podcasts and press interviews, inventorship of the manufacturing, structure and use of mRNA for vaccine purposes was long ago determined by the US Patent and Trademark office, with many US and foreign patents issued based on the work of myself (1987- 1991) and colleagues (1990-1991), all of which (including the initial invention disclosures which I solo authored) list me as an inventor. That fact is not disputed by the Nobel committee. It is extremely unusual for the Nobel Prize in Physiology or Medicine to not recognize those that originated the ideas, technology, and performed initial proof of concept. This appears to have been circumvented in this case by limiting the scope of the award to the COVID-19 mRNA vaccines themselves, and not to the discoveries and inventorship of the platform technology.
The key statement by the committee is quoted in their announcement tweet and corresponding graphic:
“For their discoveries concerning nucleotide base modifications that enabled the development of effective mRNA vaccines against COVID-19”
There are three logic elements to the statement.
“Discoveries concerning nucleotide base” refers to the incorporation of pseudouridine in place of uridine throughout an in vitro transcribed (synthetically produced) mRNA. Which results in a molecule which is quite different in many key biological properties from natural mRNA, but can still be translated into protein. The work in question is described in three publications and a patent. In contrast to my original work and patents none of which discuss use of this for vaccine development, which occurred 15 years prior to the initial publication.
“ Karikó, K., Buckstein, M., Ni, H. and Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Karikó, K., Muramatsu, H., Welsh, F.A., Ludwig, J., Kato, H., Akira, S. and Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 16, 1833–1840 (2008).
Anderson, B.R., Muramatsu, H., Nallagatla, S.R., Bevilacqua, P.C., Sansing, L.H., Weissman, D. and Karikó, K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 38, 5884–5892 (2010).”
“Enabled the development” is actually a false statement. The incorporation of pseudouridine was not an “enabling” improvement on the existing state of the art. This is demonstrated by the robust clinical (human) adaptive antibody vaccine response to the CureVac SARS-CoV-2 vaccine candidate, which did not incorporate pseudouridine. The dose (and apparently the toxicity) of the mRNA formulation employed by CureVac in their clinical tests was lower than that employed by Moderna and BioNTech, and the antibody titers were also lower. But still quite substantial. There is no evidence that antibody titer translates to clinical protection from SARS-CoV-2 infection or COVID-19 disease, but despite this fact it was widely inferred and publicized that the CureVac product was inferior. Whether or not this was the case we will never know, as further clinical testing at a higher dose was not performed- CureVac basically gave up on competing in the COVID-19 vaccine race, presumably due to lack of sufficient funds. However, there is no disputing the fact that incorporation of pseudouridine is not required for delivered mRNA to elicit an adaptive immune (antibody) response. In sum, the claim that pseudouridine incorporation is an “enabling” improvement on prior art (that being patent and inventorship language) is clearly false for this reason.
“Effective mRNA vaccines against COVID-19” is a very controversial statement. The basis cited by the committee for this assertion is included in the corresponding press release (attached below). “Protective effects of around 95% were reported” refers to the initial interpretation of the clinical trial results obtained, both the interpretation of which and the trials themselves have since been shown to have been fraudulent. This statement is not consistent with current understanding of those trial results, but rather reflects initial propaganda subsequently demonstrated to be highly misleading. “The vaccines have saved millions of lives and prevented severe disease in many more” is also not fact based, but rather is also often repeated propaganda. This assertion is largely based on the difference between initial analysis and modeling data suggesting a 3.4% case fatality rate for SARS-CoV-2 infection, subsequently shown to have been highly inflated, and the current best estimates of case fatality rates which are in the range of a fraction of 1%. This difference has been asserted to be the consequence of vaccine efficacy, but this is not supported by actual clinical epidemiology data. Current analyses based on all cause mortality data actually indicate either no effectiveness of the vaccines or negative effectiveness (multiple studies demonstrate that the onset of vaccination campaigns (both general types and mRNA genetic types) are correlated with an increase in all cause mortality, not a decrease). In addition, data from many different databases indicate negative effectiveness for these mRNA-based products beginning from two to seven months after administration. Negative effectiveness in this case refers to the observation that recipients are more likely to be hospitalized with COVID-19 than those not receiving these mRNA - based products.
To the extent that there was a relevant enabling improvement on the work of myself and my named co-inventors from the 1987-1991 time frame, that would be the formulation advancements of Dr. Pieter Cullis and colleagues at the University of British Columbia. Not the incorporation of pseudouridine. Dr. Cullis and colleagues were not included in this award decision.
The remarkably rapid development and deployment of these mRNA products parallel and correspond to the timelines achieved by other more traditional COVID-19 vaccines (“Sputnik” series etc.). This is not the consequence of the mRNA/pseudouridine technology, but rather to the willingness of regulatory authorities worldwide to suspend normal vaccine non-clinical and clinical development processes required to assess safety and efficacy. The committee asserts that these vaccines were “approved” as early as December 2020, which is another falsehood. These products were Emergency Use Authorized via suspension of normal testing, processes and review.
The demonstrable falsehoods employed by the Nobel Prize committee to justify this award indicates that the decision was not based on scientific and medical fact, but rather reflects non-scientific and medical considerations other than those cited by the committee.
Pfizer is a major donor to the Karolinska Institute.
Abstract
Seventeen equatorial and Southern-Hemisphere countries were studied (Argentina, Australia, Bolivia, Brazil, Chile, Colombia, Ecuador, Malaysia, New Zealand, Paraguay, Peru, Philippines, Singapore, South Africa, Suriname, Thailand, Uruguay), which comprise 9.10 % of worldwide population, 10.3 % of worldwide COVID-19 injections (vaccination rate of 1.91 injections per person, all ages), virtually every COVID-19 vaccine type and manufacturer, and span 4 continents.
In the 17 countries, there is no evidence in all-cause mortality (ACM) by time data of any beneficial effect of COVID-19 vaccines. There is no association in time between COVID-19 vaccination and any proportionate reduction in ACM. The opposite occurs.
All 17 countries have transitions to regimes of high ACM, which occur when the COVID-19 vaccines are deployed and administered. Nine of the 17 countries have no detectable excess ACM in the period of approximately one year after a pandemic was declared on 11 March 2020 by the World Health Organization (WHO), until the vaccines are rolled out (Australia, Malaysia, New Zealand, Paraguay, Philippines, Singapore, Suriname, Thailand, Uruguay).
Unprecedented peaks in ACM occur in the summer (January-February) of 2022 in the Southern Hemisphere, and in equatorial-latitude countries, which are synchronous with or immediately preceded by rapid COVID-19-vaccine-booster-dose rollouts (3rd or 4th doses). This phenomenon is present in every case with sufficient mortality data (15 countries). Two of the countries studied have insufficient mortality data in JanuaryFebruary 2022 (Argentina and Suriname).
Detailed mortality and vaccination data for Chile and Peru allow resolution by age and by dose number. It is unlikely that the observed peaks in all-cause mortality in JanuaryFebruary 2022 (and additionally in: July-August 2021, Chile; July-August 2022, Peru), in each of both countries and in each elderly age group, could be due to any cause other than the temporally associated rapid COVID-19-vaccine-booster-dose rollouts. Likewise, it is unlikely that the transitions to regimes of high ACM, coincident with the rollout and sustained administration of COVID-19 vaccines, in all 17 SouthernHemisphere and equatorial-latitude countries, could be due to any cause other than the vaccines.
Synchronicity between the many peaks in ACM (in 17 countries, on 4 continents, in all elderly age groups, at different times) and associated rapid booster rollouts allows this 3 firm conclusion regarding causality, and accurate quantification of COVID-19-vaccine toxicity.
The all-ages vaccine-dose fatality rate (vDFR), which is the ratio of inferred vaccine induced deaths to vaccine doses delivered in a population, is quantified for the JanuaryFebruary 2022 ACM peak to fall in the range 0.02 % (New Zealand) to 0.20 % (Uruguay). In Chile and Peru, the vDFR increases exponentially with age (doubling approximately every 4 years of age), and is largest for the latest booster doses, reaching approximately 5 % in the 90+ years age groups (1 death per 20 injections of dose 4). Comparable results occur for the Northern Hemisphere, as found in previous articles (India, Israel, USA).
We quantify the overall all-ages vDFR for the 17 countries to be (0.126 ± 0.004) %, which would imply 17.0 ± 0.5 million COVID-19 vaccine deaths worldwide, from 13.50 billion injections up to 2 September 2023. This would correspond to a mass iatrogenic event that killed (0.213 ± 0.006) % of the world population (1 death per 470 living persons, in less than 3 years), and did not measurably prevent any deaths.
The overall risk of death induced by injection with the COVID-19 vaccines in actual populations, inferred from excess all-cause mortality and its synchronicity with rollouts, is globally pervasive and much larger than reported in clinical trials, adverse effect monitoring, and cause-of-death statistics from death certificates, by 3 orders of magnitude (1,000-fold greater).
The large age dependence and large values of vDFR quantified in this study of 17 countries on 4 continents, using all the main COVID-19 vaccine types and manufacturers, should induce governments to immediately end the baseless public health policy of prioritizing elderly residents for injection with COVID-19 vaccines, until valid risk-benefit analyses are made.
Here is the formal press release justifying this award issued by the Committee (The Nobel Assembly at Karolinska Institutet).
Press release
2023-10-02
The Nobel Assembly at Karolinska Institutet
has today decided to award
the 2023 Nobel Prize in Physiology or Medicine
jointly to
Katalin Karikó and Drew Weissman
for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19
The discoveries by the two Nobel Laureates were critical for developing effective mRNA vaccines against COVID-19 during the pandemic that began in early 2020. Through their groundbreaking findings, which have fundamentally changed our understanding of how mRNA interacts with our immune system, the laureates contributed to the unprecedented rate of vaccine development during one of the greatest threats to human health in modern times.
Vaccines before the pandemic
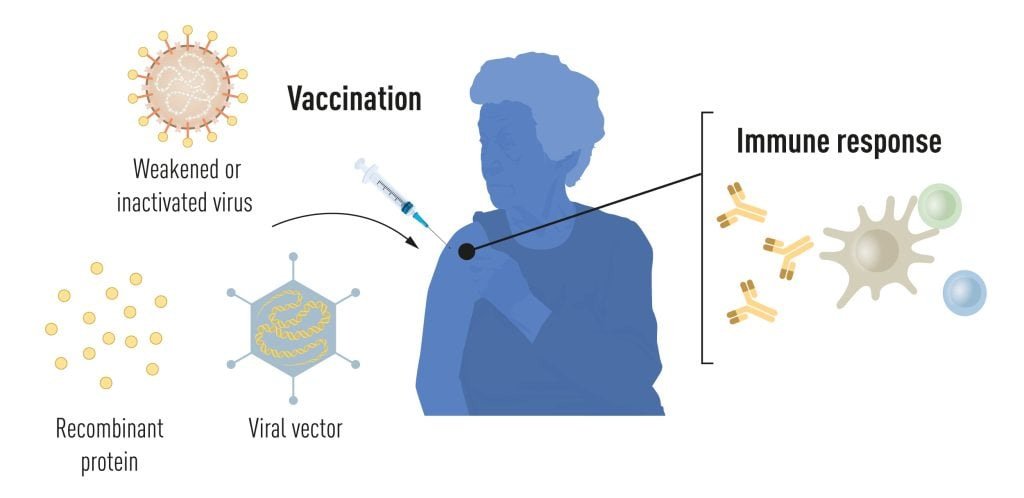
Vaccination stimulates the formation of an immune response to a particular pathogen. This gives the body a head start in the fight against disease in the event of a later exposure. Vaccines based on killed or weakened viruses have long been available, exemplified by the vaccines against polio, measles, and yellow fever. In 1951, Max Theiler was awarded the Nobel Prize in Physiology or Medicine for developing the yellow fever vaccine.
Thanks to the progress in molecular biology in recent decades, vaccines based on individual viral components, rather than whole viruses, have been developed. Parts of the viral genetic code, usually encoding proteins found on the virus surface, are used to make proteins that stimulate the formation of virus-blocking antibodies. Examples are the vaccines against the hepatitis B virus and human papillomavirus. Alternatively, parts of the viral genetic code can be moved to a harmless carrier virus, a “vector.” This method is used in vaccines against the Ebola virus. When vector vaccines are injected, the selected viral protein is produced in our cells, stimulating an immune response against the targeted virus.
Producing whole virus-, protein- and vector-based vaccines requires large-scale cell culture. This resource-intensive process limits the possibilities for rapid vaccine production in response to outbreaks and pandemics. Therefore, researchers have long attempted to develop vaccine technologies independent of cell culture, but this proved challenging.
Figure 1. Methods for vaccine production before the COVID-19 pandemic. © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
mRNA vaccines: A promising idea
In our cells, genetic information encoded in DNA is transferred to messenger RNA (mRNA), which is used as a template for protein production. During the 1980s, efficient methods for producing mRNA without cell culture were introduced, called in vitro transcription. This decisive step accelerated the development of molecular biology applications in several fields. Ideas of using mRNA technologies for vaccine and therapeutic purposes also took off, but roadblocks lay ahead. In vitro transcribed mRNA was considered unstable and challenging to deliver, requiring the development of sophisticated carrier lipid systems to encapsulate the mRNA. Moreover, in vitro-produced mRNA gave rise to inflammatory reactions. Enthusiasm for developing the mRNA technology for clinical purposes was, therefore, initially limited.
These obstacles did not discourage the Hungarian biochemist Katalin Karikó, who was devoted to developing methods to use mRNA for therapy. During the early 1990s, when she was an assistant professor at the University of Pennsylvania, she remained true to her vision of realizing mRNA as a therapeutic despite encountering difficulties in convincing research funders of the significance of her project. A new colleague of Karikó at her university was the immunologist Drew Weissman. He was interested in dendritic cells, which have important functions in immune surveillance and the activation of vaccine-induced immune responses. Spurred by new ideas, a fruitful collaboration between the two soon began, focusing on how different RNA types interact with the immune system.
The breakthrough
Karikó and Weissman noticed that dendritic cells recognize in vitro transcribed mRNA as a foreign substance, which leads to their activation and the release of inflammatory signaling molecules. They wondered why the in vitro transcribed mRNA was recognized as foreign while mRNA from mammalian cells did not give rise to the same reaction. Karikó and Weissman realized that some critical properties must distinguish the different types of mRNA.
RNA contains four bases, abbreviated A, U, G, and C, corresponding to A, T, G, and C in DNA, the letters of the genetic code. Karikó and Weissman knew that bases in RNA from mammalian cells are frequently chemically modified, while in vitro transcribed mRNA is not. They wondered if the absence of altered bases in the in vitro transcribed RNA could explain the unwanted inflammatory reaction. To investigate this, they produced different variants of mRNA, each with unique chemical alterations in their bases, which they delivered to dendritic cells. The results were striking: The inflammatory response was almost abolished when base modifications were included in the mRNA. This was a paradigm change in our understanding of how cells recognize and respond to different forms of mRNA. Karikó and Weissman immediately understood that their discovery had profound significance for using mRNA as therapy. These seminal results were published in 2005, fifteen years before the COVID-19 pandemic.
Figure 2. mRNA contains four different bases, abbreviated A, U, G, and C. The Nobel Laureates discovered that base-modified mRNA can be used to block activation of inflammatory reactions (secretion of signaling molecules) and increase protein production when mRNA is delivered to cells. © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
In further studies published in 2008 and 2010, Karikó and Weissman showed that the delivery of mRNA generated with base modifications markedly increased protein production compared to unmodified mRNA. The effect was due to the reduced activation of an enzyme that regulates protein production. Through their discoveries that base modifications both reduced inflammatory responses and increased protein production, Karikó and Weissman had eliminated critical obstacles on the way to clinical applications of mRNA.
mRNA vaccines realized their potential
Interest in mRNA technology began to pick up, and in 2010, several companies were working on developing the method. Vaccines against Zika virus and MERS-CoV were pursued; the latter is closely related to SARS-CoV-2. After the outbreak of the COVID-19 pandemic, two base-modified mRNA vaccines encoding the SARS-CoV-2 surface protein were developed at record speed. Protective effects of around 95% were reported, and both vaccines were approved as early as December 2020.
The impressive flexibility and speed with which mRNA vaccines can be developed pave the way for using the new platform also for vaccines against other infectious diseases. In the future, the technology may also be used to deliver therapeutic proteins and treat some cancer types.
Several other vaccines against SARS-CoV-2, based on different methodologies, were also rapidly introduced, and together, more than 13 billion COVID-19 vaccine doses have been given globally. The vaccines have saved millions of lives and prevented severe disease in many more, allowing societies to open and return to normal conditions. Through their fundamental discoveries of the importance of base modifications in mRNA, this year’s Nobel laureates critically contributed to this transformative development during one of the biggest health crises of our time.
If you would like these blog posts to appear in your email stream,
please sign up to