SARS-CoV-2 Variant HV.1; Obsolete Boosters
No evidence that current boosters are "safe and effective" against dominant HV.1 variant
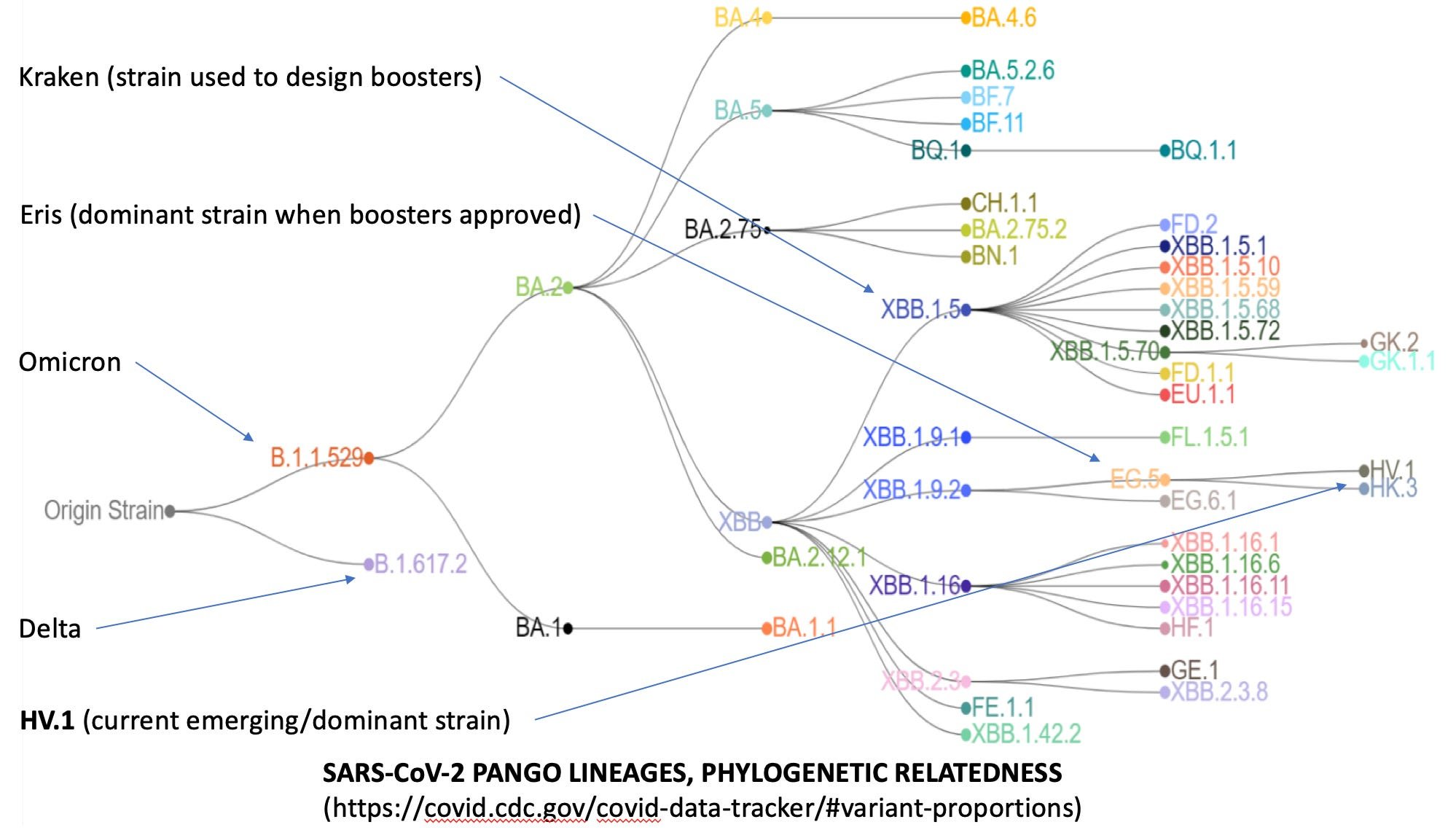
As previously covered (here), the currently available FDA emergency use authorized “booster vaccines” were designed based on recommendations developed at the FDA VRPBAC (vaccine and related products biological advisory committee), which predicted that the dominant SARS-CoV-2 variant this fall would be the “Kraken” (XBB.1.5) viral variant of Omicron.
To re-cap, the FDA and it’s advisory committee are operating based on the hypothesis that the dominant mechanism of protection against SARS-CoV-2 infection, spread, and COVID-19 disease afforded by vaccination or natural immunity involves “neutralizing antibodies”. This hypothesis is unproven, and no immunologic “correlate of protection” which predicts protection from either infection or disease has been clinically proven. To the extent that high levels of human “neutralizing antibodies” from the SARS-CoV-2 genetic “vaccines” provide any protection, they seem to “correlate” to reduced disease severity for some period after dosing, but do not prevent infection, replication, spread, disease or death.
Despite this undeniable fact, the FDA has disregarded its decades long policies concerning the requirement to validate a “correlate of protection” test before it can be used as a surrogate for actual clinical demonstration that a “vaccine” product will protect against infection, replication, spread, disease or death. Until so proven, historically a vaccine developer has been forbidden from making any claim of protection unless that claim is demonstrated and statistically ”validated”. Validation requiring some sort of well controlled human clinical trials. Alternatively, a vaccine developer can create a well-controlled “correlate of protection” laboratory test that employs human samples, and statistically demonstrate that it predicts (correlates) a relevant clinical outcome (ergo preventing infection, spread, severe disease of death). Only then can such the outcomes of such a test be used as a surrogate endpoint in clinical trials, rather than actually clinically testing whether the product has a significant impact on the intended endpoint.
Influenza vaccine development provides an example of this. In the case of influenza, a laboratory test called the hemagglutination-inhibition (HAI) test measures solutions of antibodies from patients that have a particular characteristic (laboratory clumping of red blood cells). With standard injected influenza vaccines, it has been statistically demonstrated that when a new vaccine is sufficiently potent at generating high enough levels of HAI antibodies (referred to as antibody “titer”), it will protect (to a significant degree) from clinical influenza disease. The nasal spray influenza vaccine (Flumist) did not meet the HAI lab test criteria, and so the developers at MedImmune were required to perform time consuming, expensive actual clinical trials to demonstrate that it protected against clinical disease. That was the way things used to be at the FDA, up until 2021 and the COVIDcrisis.
The process of statistical “validation” of a surrogate “correlate of protection” is rigorous, technically challenging, expensive, and time consuming. In the case of SARS-CoV-2, the CDC and FDA have arbitrarily decided to throw away normal regulatory standards and allow the substitution of non-validated assays as being predictive of efficacy or effectiveness of the COVID vaccines. Instead of taking the time to develop and statistically validate a test, they have relied on the multiple studies indicating that human “neutralizing antibody” levels are loosely associated with reduced disease severity, none of which have met criteria for statistical validation of that endpoint. All of these studies have used blood samples and lab test “titer” results averaged across many (human) patients, and the FDA now considers unvalidated “neutralizing antibody” tests to be generally correlated with short term protection against disease severity. The arbitrary and capricious use of such non-validated laboratory assays as a substitute for actual clinical trials proving that these products will perform as advertised has apparently been justified on the basis of expediency - the virus is evolving too fast to allow manufacturing and rigorous testing of these products before they are made obsolete by new viral variants.
Just to re-cap, the whole rationale for using Emergency Use Authorization to jam the modified-mRNA vaccine technology past the normal regulatory processes designed over decades to ensure safety and effectiveness was so that these products could be produced so rapidly that Pharma could promptly field effective vaccine products to protect the public from new viruses. Clearly, the technology and the EUA process bypass have failed to meet mission requirements for anti-viral effectiveness. And just as clearly the regulators have also thrown caution about vaccine product safety and adulteration to the wind. It is long past time to acknowledge that the most massive human experiment in the history of the world has failed.
The CDC Morbidity and Mortality Weekly Report (MMWR), a non-peer reviewed internal “official” publication of the CDC, has published a review article summarizing the data justifying the use of neutralizing antibody lab tests as a surrogate for clinical trials designed to actually prove vaccine protection, titled “Correlates of Protection, Thresholds of Protection, and Immunobridging among Persons with SARS-CoV-2 Infection”. The research reviewed focuses on the use of human sample testing, and includes the following conclusions:
The work on the vaccine-comparison model approach has so far shown that this model, which was originally calibrated on data for ancestral SARS-CoV-2 infections, can also be used to predict vaccine effectiveness against SARS-CoV-2 variants and after boosting, as long as one adjusts for the drop in neutralization titers to the variants and rise in neutralization after boosting (12,18,19; D. Cromer et al., unpub. data). However, the need to standardize neutralization assays for SARS-CoV-2 variants presents an ongoing challenge.
In vitro neutralizing antibody titers against SARS-CoV-2 present a clear correlate of protection from symptomatic SARS-CoV-2 infection. Studies of passive administration of neutralizing monoclonal antibodies in animals and humans support that neutralizing antibody titers are a mechanistic correlate of protection (21–23). Indeed, a recent study comparing protective titers in prophylactic and therapeutic studies suggests that the protective titers may be very similar (E. Stadler et al., unpub. data, https://www.medrxiv.org/content/10.1101/2022.03.21.22272672v2External Link). Neutralizing antibody levels are also correlated with protection from severe SARS-CoV-2 infection (2).
In conclusion, our findings show that the different COVID-19 correlate of protection studies, which seemingly report different thresholds of protection, have strong agreement. However, other immune responses may also play a substantial role in protection against progression from symptomatic to severe SARS-CoV-2 infection.
When you cut through the obfuscation, what the CDC authors are saying is that there does not appear to be agreement across the various publications concerning what levels of “neutralizing antibodies” are protective, but if you apply a statistical adjustment method, they can make the studies agree with each other. Which is probably why this is published in the CDC’s in-house, non-peer reviewed journal MMWR.
The publication basically asserts that an established viral neutralization assay can be useful in comparing the protection from disease or severe disease associated with different vaccine products (using human blood samples and statistical adjustments). However, as the virus evolves to new variants it becomes difficult to predict effectiveness of “vaccines” against these new variants. Furthermore, the publication indicates that predictive correlation of neutralization with infection (as opposed to disease) is much more problematic, and acknowledges that other immune responses (such as those elicited by natural infection) are likely to also play “a substantial role” in protection from disease progression.
So where does this leave the FDA and CDC in their efforts to justify Emergency Use Authorization of these products (disregarding the inconvenient fact that there is no emergency)? In the case of the currently available “booster” products, emergency use authorization was granted based on a “neutralizing antibody” titer test using blood (serum) from a small number of inoculated mice. Despite the exclusive reliance on human samples for this and virtually all other peer reviewed publications addressing the (unvalidated) correlation between “neutralizing antibody titers” and reduction in COVID-19 disease severity? How dosing equivalence between mice and man was actually determined was not shared with the likes of you and I. And I can assure you that is a very problematic calculation. Let alone whether said mice had been previously injected with prior versions of the vaccine. And overlooking how a hundred or more other immunologic variables which differentiate mice and man were (not) controlled for. One is left wondering if anyone in the decision making loop at FDA and CDC has any experience at all in immunology and vaccine research.
You see, the FDA and CDC had a problem. They were clearly told by their bureaucratic masters to get these “boosters” approved for the fall, but (as noted in the graphic above) they were designed to protect against the fearsome Kraken variant, but by the time they were ready for being jammed through EUA approval the Kraken (XBB.1.5) had become extinct, out competed by Eris (EG.5).
And as can be seen from the above phylogeny diagram, these two are on separate branches of the XBB tree. So what’s a regulator to do? Simple: rely on making vague, unsupported statements based on mouse serum cross neutralization assays, to the effect that the cross reactivity looks good enough to you. And know that since you are the expert (per Chevron deference), you can never be held accountable in the courts for your actions. And for extra protection against having your sloppiness and misdeeds detected, do not bother to allow anyone else to review the data. And make sure to activate CDC, DHS, and CISA tools and contractors to censor and defame anyone who has a different opinion. After all, the FDA and CDC authorities have had a perfect record of predicting what strategies and products will and will not work for preventing and treating COVID-19. Because, as was clearly stated in the Netflix 2023 remake version of the “Treasure of the Sierra Madre”, the FDA don’ need no stinkin’ data.
Please keep in mind that most of the rest of the word is done with this merry go round of booster de jour, but here in the good old USA, the FDA and CDC (and DHS, and CIA, and DoD) somehow think that the declining market capitalization of Pfizer and Moderna represent an existential threat to both public health and the US Biopharmaceutical-defense complex, and so we need COVID boosters forever.
And you thought that MK-Ultra’s Dr. Strangelove (whose real name was Dr. Gottleib, by the way) was insane?
Unfortunately, despite the collective wisdom of the US federal administrative state, SARS-CoV-2 is evolving to escape antibody-based protection much faster than the geniuses at HHS, Pfizer and Moderna can keep up, perhaps in part due to injection of “billions of doses” of “safe and effective” (and incredibly leaky) “vaccines”.
Unfortunately, according to the official propaganda outlet for the US Government (PBS), for some reason the US public is not responding with great enthusiasm to all of the federal government and Pharma deployed PsyOps and marketing designed to get them to take this particular jab. Perhaps the general public is smarter than the bureaucrats think, and are aware that mice are not men. Only 7% of adults and 2% of children are booster jabbed at this point. And something tells me that even those data are strongly biased in favor of vaccine uptake in bicoastal urban centers relative to flyover states.
Which, as an aside, raises the question of which regions and states are smarter. Or perhaps wiser. Or just more resistant to cooperative public-private PsyOps campaigns. But of course correlation does not prove causation.
Which brings us to the present, and the new SARS-CoV-2 variant with the “Pango” designation of HV.1.
Seeing how fast the evolution of SARS-CoV-2 is moving, did the geniuses at FDA, CDC, Pfizer, or Moderna (who seem unable to read a simple plasmid DNA sequence or understand the risks of injecting people with huge amounts of DNA fragments formulated into lipid nanoparticle delivery agents) think ahead? Did they think that, as they sought to jab the entire population of the USA once again, that the virus would pause its evolution for a bit, sort of take a biobreak for a moment? Did they even spare one moment to consider that deploying a mismatched “booster” vaccine would further compound the proven adverse event risk for each jabbed individual man, woman and child?
Or, in their mad rush to prevent the apocalyptic surge of death and disease that Pfizer exec and CIA consultant Dr. Scott Gottleib had predicted for this fall, did they consider that even the crummy mouse blood tests they had relied upon would not address the new variants which Mother Nature, or God, or some biolab full of CIA-funded mad scientists located in some backwater country were going to toss into the mix? Well, apparently not. But here we are.
Eris is so yesterday, and has apparently evolved (or was engineered) into a more infectious new kid in town designated HV.1 (CDC data below). Which is even more mismatched to the Kraken booster jabs than Eris was.
Just in case you were wondering how this shift from Eris to HV.1 is going in your region, here is a plot of the current geographic distribution of variants.
And for for those from Missouri who insist on being shown the data, to provide just a bit of reality testing and recalibration concerning the risk of infection currently associated with SARS-CoV-2, here is a plot of how this is all playing out on the global stage (CDC has stopped reporting US national data for some reason):
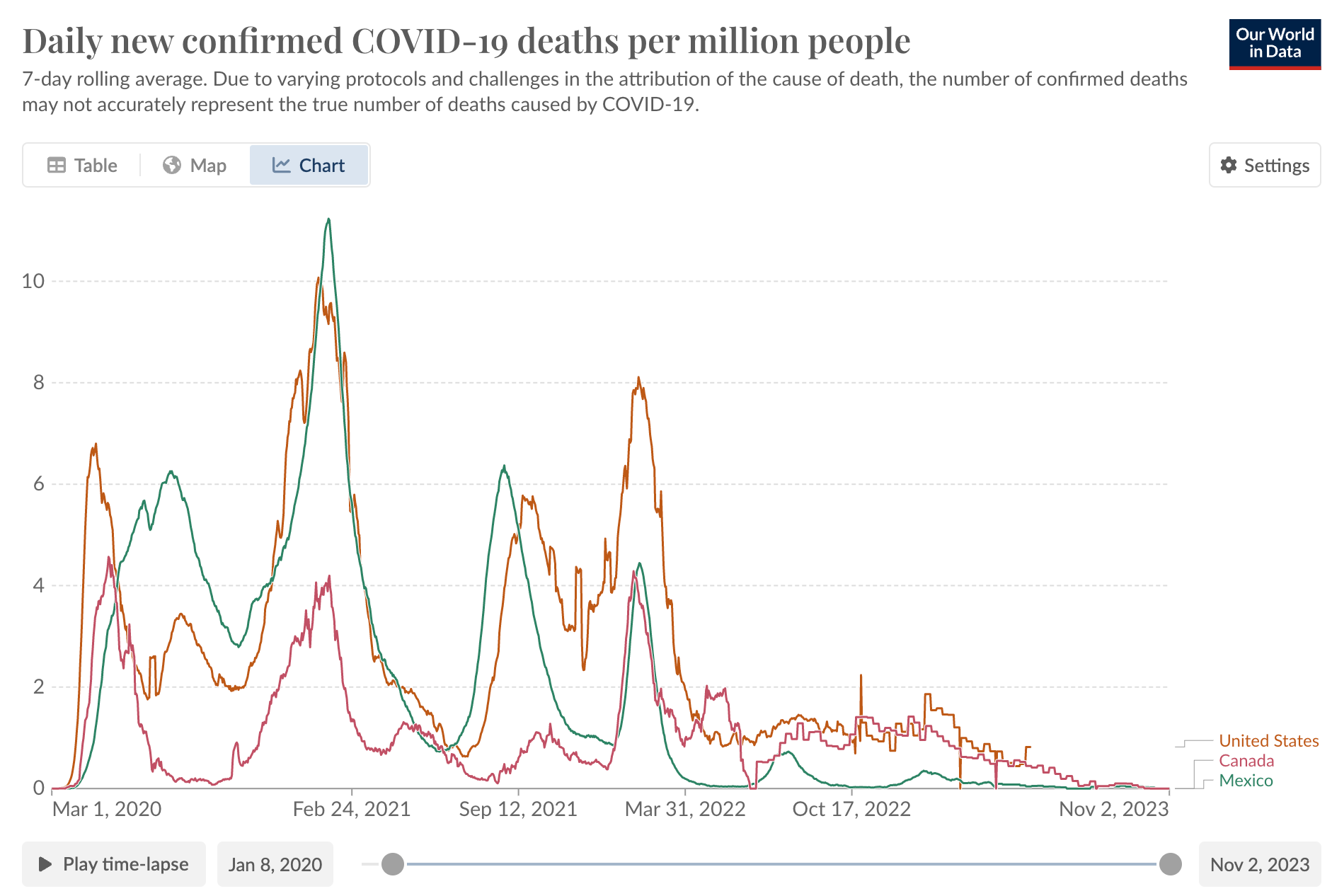
Although the CDC has stopped sharing data, a reasonable extrapolation can be made by looking at what is going on in our regional neighbors to the north and south of the USA.
So those are the case counts. What about deaths (adjusted for population)?
Hard to make the case that we are facing a COVID-19 public health emergency at this point.
Getting back to HV.1, what are the new mutations and why should you care? Are we all going to die, as a certain veterinarian scientist keeps predicting? Well, yes, that is generally how things work, but most likely not from this or any other variant.
I think that Korin Miller, writing for the alternative health news site Prevention.com has done a great job objectively summarizing key points for the layperson:
What Is HV.1? Experts Explain the Latest COVID Variant
Experts say it’s highly contagious—but should you be worried?
As we move into the thick of respiratory virus season, there’s a new COVID-19 variant to keep your eye on: HV.1. Cases of this variant are increasing at a rapid pace in the U.S., and it seems to be on track to replace the current dominant variant, EG.5.
Data from the Centers for Disease Control and Prevention (CDC) show that HV.1 surfaced in mid-summer, before cases began rapidly increasing in September. Now, the variant causes nearly 20% of all COVID-19 cases in the country.
But what is HV.1 and how worried about it should you be? Here’s what we know right now.
What is COVID-19 variant HV.1?
Like many COVID-19 variants that have surfaced recently, HV.1 is related to the Omicron strain, says Amesh A. Adalja, M.D., infectious disease expert and senior scholar at the Johns Hopkins Center for Health Security. “HV.1 is another Omicron XBB variant that has descended from EG.5,” he says. Meaning, HV.1 came from EG.5, which came from XBB, which is a form of Omicron.
“It’s yet another Omicron variant,” says Thomas Russo, M.D., professor and chief of infectious disease at the University at Buffalo in New York.
HV.1 has several changes to its spike protein from EG.5, which is what SARS-CoV-2, the virus that causes COVID-19 uses to latch onto your cells and make you sick, Dr. Russo explains.
How contagious is HV.1?
It’s difficult to say at this point. However, Dr. Russo points to its rapid rise (from 0.5% of cases in mid-July to nearly 20% of cases in mid-October) as an indication this variant is pretty contagious.
“HV.1’s most recent claim to fame is the rapidly increasing proportion of cases in the U.S.,” he says.
Should I be concerned about HV.1?
The experts we spoke with aren’t alarmed about HV.1 at this point. “It is important to recognize that there will always be new variants of SARS-CoV-2, just as there are with any other endemic respiratory virus and most will not be of concern to anyone,” Dr. Adalja says.
While HV.1 is “very transmissible,” it also doesn’t seem to be causing more serious disease than other variants circulating, says William Schaffner, M.D., an infectious disease specialist and professor at the Vanderbilt University School of Medicine. “I don’t think people should be very concerned about this,” he says.
What are the symptoms of HV.1?
The symptoms of HV.1 are consistent with other COVID-19 symptoms so far, Dr. Russo says. According to the CDC, those include:
Fever or chills
Cough
Shortness of breath or difficulty breathing
Fatigue
Muscle or body aches
Headache
New loss of taste or smell
Sore throat
Congestion or runny nose
Nausea or vomiting
Diarrhea
In general, HV.1 seems to cause more cold-like symptoms than anything, Dr. Schaffner says. “Some of the symptoms recorded have been cough, fatigue, congestion, and a runny nose,” he says. “That sounds pretty much like the common cold.”
For the more detail oriented
(quoted from the Epoch Health article “New COVID Variant 'HV.1' Has Surprising Mutations”)
HV.1 shares almost all spike mutations that EG.5 carries, including F456L. However, HV.1 has one surprising additional mutation: L452R. The L452R mutation, which increases infectivity, is one of the key mutations of the delta variant in 2021 but is absent in the Omicron variant.
HV.1 is believed to have slightly better transmissibility than the previous dominant strain EG.5, as its binding affinity to the ACE2 receptor is modestly better than EG.5's, according to a recent analysis conducted by Peking University assistant professor Yunlong Cao and his team.
HV.1 More Easily Evades Vaccines
HV.1 can further render the current COVID-19 vaccines ineffective, as it is even better able to escape vaccine-induced immunity than EG.5. This means none of the current COVID vaccines can induce any effective antibodies to bind HV.1.
This results from the variant's key mutations F456L, L452R, F157L, and Q52H. These latest mutations indicate that the new omicron variants have developed cunning strategies to evade our manmade vaccines by explicitly targeting specific sites. By doing so, they may be able to partially or wholly escape the protective effects of vaccination, potentially leading to breakthrough infections among the vaccinated.
The virus' faster transmissibility and improved evasiveness help explain the rising curve we observe in the CDC report <the graphical data cited above>.
Not surprisingly, HV.1 has shown a further reduced antibody-neutralizing response, increasing the chances of reinfection or breakthrough infections, according to Mr. Cao's experimental data.
What does this mean for the boosters?
My personal assessment is that, at this point, those who have accepted the boosters have also accepted significant risk of developing a wide range of adverse events with essentially no practical benefit. I will be glad to revise that opinion when the CDC or FDA share data demonstrating that the current boosters provoke levels of neutralizing antibodies directed against HV.1 which are consistent with the levels predicted to correlated with reduction in disease risk (see above MMWR publication). But no more mouse bloods please. Lets see human serum neutralization tests against the HV.1 virus isolate.
Until those data are made available and are sufficiently compelling, what I see is all risk and virtually no evidence of benefit associated with these hastily “Emergency Use Authorized” products designed to mitigate risk of a fall public health crisis which is not coming to pass.
If you are in the 20% of the population who desperately need some sort of personal reassurance to alleviate the intensive anxiety which you are experiencing consequent to exposure to the corporate media/USG propaganda machine, I guess you might as well wear a paper dust mask. Just for the psychological reassurance. As I am seeing so many from major bicoastal urban centers once again turning to. At least the risk of life threatening adverse events from paper dust masks will be lower than with the jab.
But for those who are in the 20% of the population that are highly resistant to the rampant PsyWar COVIDcrisis propaganda, and all those many in the persuadable middle who have been passively opting out of this round of “boosters’ for you and/or your children, I hope that the above data provide reassurance that you can go buy a nice dinner out for your significant other with the money you save by not going down to your local chain pharmacy to get the latest jab.
If you would like these blog posts to appear in your email stream,
please sign up to